
Soil Tests

When to do basic soil test
Soil samples can be taken at any time of the year, but it is best to sample the soil a couple of months before fertilizing the lawn or establishing perennials, or before planting a garden, to allow ample time for the lime to react with the soil.
Soil samples can be taken in the spring or fall for established sites. For new sites, soil samples can be taken any time that the soil is workable. Most companies or people conduct their soil tests in the spring. However, fall is the preferred time for taking soil tests if one wants to avoid the spring rush and suspects a soil pH problem. Fall soil testing will allow you ample time to apply lime if the soil pH is too low. Sulfur should be applied in the spring if the soil pH is too high.
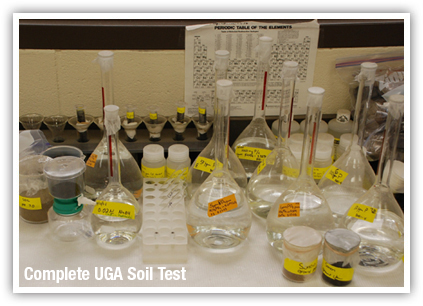
The testing process
For a soil analysis, we collect twelve or more cores which will then be combined into one composite sample. The samples will include soil from the surface to a depth of 2-4 inches. We place the samples in a clean cup and mix them thoroughly (yummy!). We take a minimum of two cups of soil per sample to test. We also keep track of which part of your yard the sample came from.
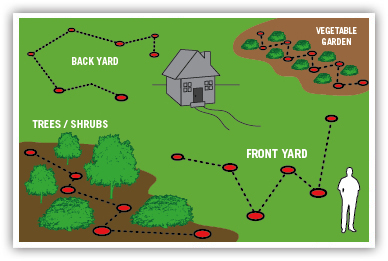
We take a soil sample from each section of your yard. Usually this means, for example, one sample in your front yard, each side yard, and the back. If you have a problem area where plants do not seem to grow well, we take a separate soil sample from that location. Each sample represents only one soil type or area for each of the locations.
Soil test results usually take between 1-2 weeks to be analyzed. You will be contacted with the results and supplied a copy of the report. We will go over the information and make recommendations accordingly.
Soil Education
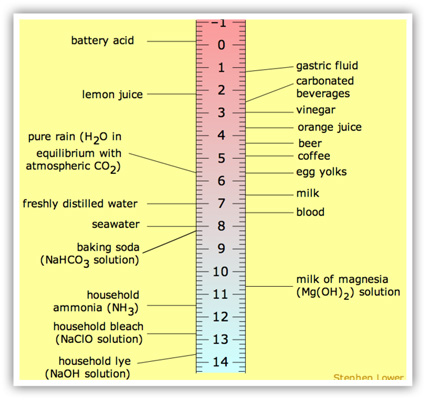
Why is (PH) levels so important in Georgia?
It’s because soil acidity, or alkalinity, directly affects all plant growth. If a soil is too sour or too sweet, plants cannot take up nutrients such as nitrogen (N), phosphorus (P), and potassium (K). And plants need specific amounts of those compounds—just like we need proteins, carbohydrates, and vitamins to grow—to thrive and fight off disease and stress. Nutrient uptake and pH
Plant roots absorb minerals such as nitrogen and iron only when they are dissolved in water. Now, if this soil "soup" solution (the mixture of water and nutrients in the soil) is too acid or alkaline, some nutrients won't be dissolved, and as a result, they are unavailable to plants. They are said to be "locked up."
To put it another way, if the pH isn’t close to what these plants require, some nutrients, such as phosphorus, calcium and magnesium, can’t be dissolved in water. And since plants drink their food instead of eating it, if the nutrients aren’t dissolved first, the plant can’t absorb them. Thus, your lawn and garden won't grow or produce to their full potential.
How the pH scale works
Like the Richter scale that's used to rate earthquakes, the pH scale is not linear. The intervals between numbers are logarithmic, which means every number on the scale shows ten times less pH concentration than the number below. Soil with a pH of 5 is ten times more acidic than soil with a pH of 6.
Soil summary in a nutshell:
Soil is like the human body. Too much salt is harmful, and it needs just the right amount of water. If it’s too sour or too sweet, nobody likes to live around it. When its chemical balance is out of whack, it suffers wear and tear. It needs testing by professionals on a regular basis. It’s likely to prefer drinking over eating, and too much P is definitely a detriment!
Interpreting your soil test results from Weed Pro® Lawn Care.
pH
An excess or deficiency of any one of the nutrients in the soil can increase or decrease the pH reading. Calcium, magnesium, potassium, and sodium all have an effect on the alkalinity of a soil. Our goal is to balance the soil in a way that will, in turn, bring the pH closer to 6.5. A pH of 6.5 is desired because this is the level at which most nutrients are readily available.
Phosphorus (P)
Phosphorus is an essential element and is classified as a macronutrient because of the relatively large amounts of P required by plants. Phosphorus is one of the three nutrients generally added to soils in fertilizers. One of the main roles of P in living organisms is in the transfer of energy. Organic compounds that contain P are used to transfer energy from one reaction in order to drive another reaction within cells. Adequate P availability for plants stimulates early plant growth and hastens maturity. Although P is essential for plant growth, the mismanagement of P in the soil can pose a threat to water quality. The concentration of P is usually sufficiently low in fresh water so that algae growth is limited. When lakes and rivers are polluted with P, excessive growth of algae often results. High levels of algae reduce water clarity and can lead to decreases in available dissolved oxygen as the algae decays, conditions that can be very detrimental to game fish populations.
Potassium (K)
The desired value of potassium is 5%, but we like to see it closer to 7% for lawns and trees. Potassium is the most critical factor in a lawn's ability to withstand wear and tear. In trees, potassium helps give stalk strength. Potassium has been referred to as the poor man's irrigation, because potassium will help a plant through droughts more than any other nutrient. Calcium (Ca) We use sulfur to drive calcium down. A high level of Ca takes up a majority of the space in the soil, preventing the efficient uptake of other nutrients. Calcium is needed to feed the microorganisms and affects the permeability of plant cell walls and the thickness of stems.
Magnesium (Mg)
Magnesium is important for photosynthesis and acts as the glue in a soil. Too much Mg in a soil causes compaction and tightness, creating major problems. The desired percentage is 11%. Many soils have a low Mg level at around 4% - 7%. The actual amount of Mg in a soil can be figured by subtracting 69% from the percentage of Ca found in the soil test, then adding that figure to the Mg percentage. This demonstrates the amount of Mg that is being tied up by Ca and therefore unavailable to a growing plant. But until Mg is above 11%, the Mg will be a limiting factor as a plant nutrient.
Sodium (Na)
Normally, sodium is not a concern. Some neighborhoods have salt problems created by incorrect watering, which causes the salt to accumulate. Watering for short periods of time frequently can cause a sodium build-up. We recommend one inch of water per week, without causing excessive runoff. This schedule will flush the harmful salts out of the root zone. Major problems arise when the level of sodium is higher than the potassium. Sodium and potassium are similar elements and plants will take up the sodium instead of the potassium, causing cells in the plant to die.
Nitrogen (N)
A calculated value from the release of humus. Nitrogen is necessary for the formation of every cell. A large amount of available nitrogen—such as found in synthetic fertilizers—causes the humus to burn out and creates rapid unhealthy growth in plants.





